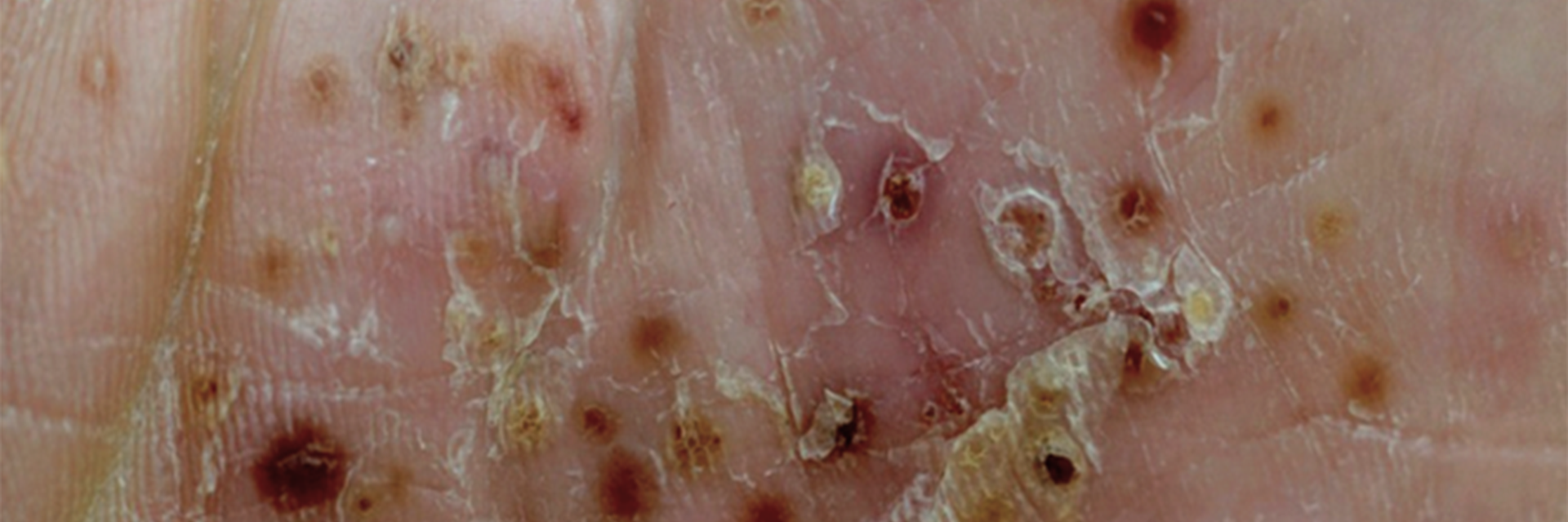
Deucravacitinib for the Treatment of Palmoplantar Pustulosis
Not Recruiting
18-100 years
All
Phase
N/A
9 participants needed
1 Location
Brief description of study
The purpose of this study is to determine if individuals diagnosed with palmoplantar pustulosis (PPP) would benefit from treatment with Deucravacitinib.
Detailed description of study
This is an open label study, and all participants will receive Deucravacitnib (SOTYKTU) for up to 24 weeks. Study visits will occur at the Perelman Center for Advanced Medicine. Participants will be compensated up to $720 for their time, depending on the number of visits completed.
Eligibility of study
You may be eligible for this study if you meet the following criteria:
- Conditions: dermatology, palmoplantar pustulosis, PPP, Palmoplantar psoriaisis
-
Age: Between 18 Years - 100 Years
-
Gender: All
The following is required to be included in this study:
- Dermatologist-confirmed diagnosis of PPP for at least 6 months
- Moderate-severe PPP
- Inadequate response to topical therapy and a candidate for systemic or phototherapy
- Willing to discontinue current topical and/or systemic PPP treatments, except for OTC emollients
Updated on
29 Oct 2024.
Study ID: 854018